It was not so long ago that cardiologists were warning us to avoid testosterone as they believed that it increased the risk of heart disease. Those original studies have been dismantled and shown to be inaccurate as they were poorly constructed and poorly interpreted. Newer literature has at every turn shown us that testosterone is a tremendous key to advancing cardiovascular health. Hypogonadism that results in suboptimal testosterone levels is very dangerous as it contributes to higher risk of diabetes, hyperlipidemia, hypertension, vascular plaque, Metabolic Syndrome and myocardial infarction. This article presents and explores some of the notable research that needs to be instilled into our cardiovascular decision making.
Of significant interest is the observation that testosterone levels are dropping in our general civilization. From the 1980s, thru the 1990’s and into the 2000’s we have seen serial drops in testosterone levels over time for all men of all ages. The Travison study exposed this 10 years ago and we need to explore why this is occurring as it contributes to our general cardiovascular risk.1
The study by Travison measured testosterone levels in 3 different groups of healthy males age 45 to 80 from 1987 through 2004. They demonstrated an alarming trend in testosterone reduction that was age-independent. This represents a population-based decline, not just the typical testosterone decline that we see with aging. They measured an approximate 22% decline in overall testosterone production across all age groups. The question becomes why are we seeing this drop in testosterone?
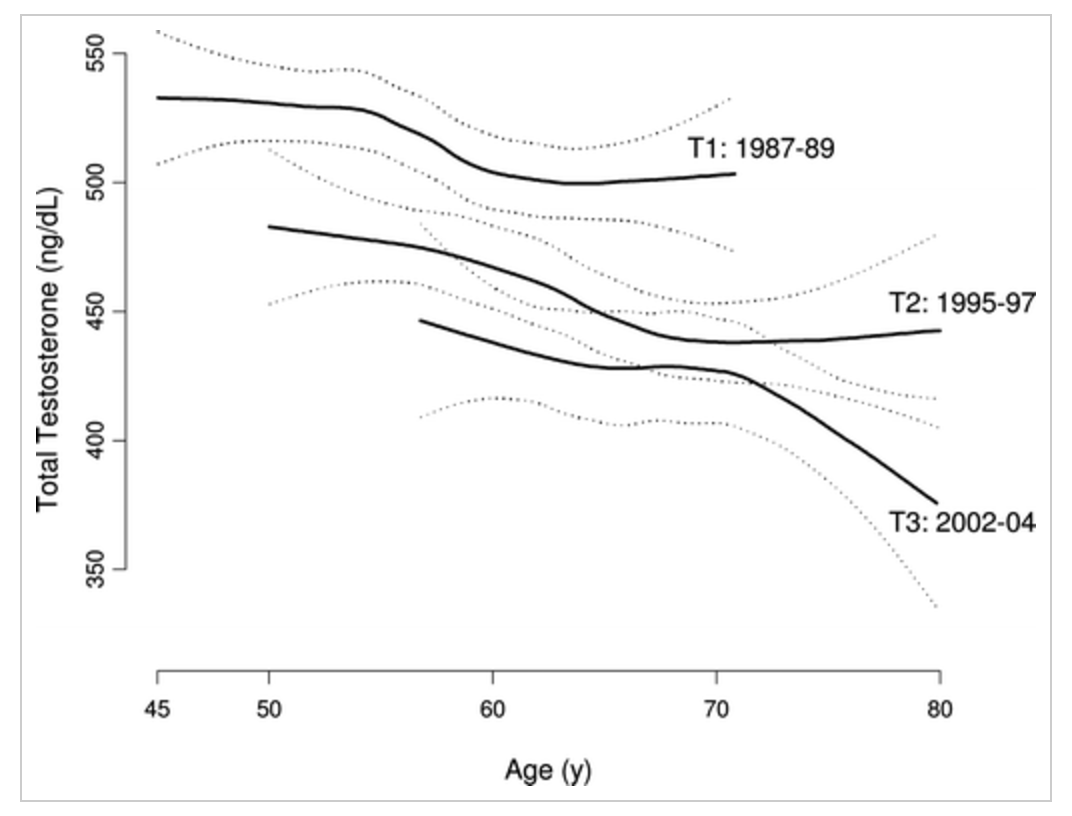
Graph from Travison article demonstrating testosterone trends in three groups of men from 3 different decades.
There are a host of reasons that may explain why testosterone levels are dropping:
- BPA, Toxins & Pesticides2-11
- Arrest Hypothalmic & Pituitary function and interfere with receptors
- BPA is correlated with every sexual dysfunction imaginable
- Polypharmacy
- Increased drug use results in “poisoned” physiology
- U.S is 5% of the world population yet we ingest 50% of all pharmaceuticals made.
- Stress – HPA-G Axis
- Cortisol is adversarial to Testosterone
- Sleep12, 13
- Testosterone is victimized by short sleep cycles
- Testosterone and REM sleep are restorative and directly correlate
- Obstructive sleep apnea damages testosterone production
- Weight Gain & Sugar14-20
- Obesity and a high fat diet poisons the Leydig cells, reducing testosterone levels.
- High glycemic diet reduces testosterone 25%
As more men trend towards this problematic fate we need to ask if this drop in testosterone is really a problem or just a simple observation? The amount of literature exploring this is rather robust and shows a clear picture that hypogonadism is not only correlated with inflammatory vascular disease but is causative in the progression to coronary disease.
Consider this simple paradigm:
- CRP is predictive of diabetes & cardiovascular events21
- CRP is consistent with obesity and inflammation
- Elevated CRP is predictive of low testosterone and testosterone replacement will reduce CRP and reduce obesity22
- Testosterone lowers blood sugar and improves vascular health
- Testosterone lowers risk for Metabolic Syndrome and cardiovascular events
The simple paradigm becomes “inflammation drives coronary disease”. Anything that increases inflammatory cytokines will work to reduce testosterone levels. The corollary is that the application of testosterone in a low androgen state will suppress and reverse the inflammatory state.23
In 2013, Zhang looked at CRP-hs and testosterone levels in 1989 healthy men between the ages of 20 and 69.22 These men did not have coronary disease, stroke history, cancer, Rheumatoid illness, thyroid or renal disease. Divided into quintiles for testosterone and SHBG he demonstrated a linear inverse relationship between CRP and testosterone levels. As testosterone goes up, CRP goes down.
Administration of IL-6 to healthy men suppressed testosterone levels. Inflammatory cytokines have an inhibitory effect on leydig cell steroidogenesis, altering 3β-hydroxysteroid dehydrogenase. In the study by Malkin, he demonstrated that testosterone replacement resulted in a significant reduction in inflammatory cytokines. TNFa dropped 50% and IL—1b dropped 37% while anti-inflammatory cytokine IL-10 rose 17%.24 Kalinchenko’s study confirmed this finding and showed that testosterone replacement significantly decreased CRP, and the inflammatory cytokines IL-1β, and TNF-α in hypogonadal men with Metabolic Syndrome.25
We know from other studies that CRP is a much more reliable predictor of coronary risk than simple LDL levels.21
The simple construct is that inflammatory lifestyle drives a rising CRP and this in turn increases coronary risk, while this same lifestyle directly contributes to hypogonadism. Heart disease is the product of inflammation:
- Athersclerosis is a disease of chronic inflammation
- TNFa & IL-1b are mediators of atheromatous plaque
- IL-10 is anti-inflammatory, inhibits atherosclerosis26-28
- IL-10 improves prognosis following an acute coronary event
These factors all improve under the influence of testosterone. The replacement of testosterone consistently reduces CRP and reverses coronary risk. In fact, in patients with coronary artery disease, testosterone deficiency is associated with poor outcomes associated with heart failure and has a significant negative impact on survival.29
Diabetes, Hypertension & Lipids
Hypertension, diabetes and hyperlipidemia are used to predict those at risk for coronary events and occupies the majority of the traditional efforts to halt the occurrence of coronary disease. So what does the literature tells us about these three issues as they relate to testosterone?
Diabetes Mellitus & Testosterone
There is ample literature proving testosterones reduces the risk of developing type 2 diabetes as well as its ability to help reverse this condition. The study by Pitteloud
looked at 60 men and assessed their mitochondrial function and expression of oxidative phosphorylation genes in skeletal muscle.30 Those with low testosterone had a reduction in ATP production thus altering glucose control. There is a subset of 34 genes responsible for oxidative phosphorylation and the rate limiting gene, UQCRB (ubiquinol-cytochrome-c reductase binding protein) is testosterone dependent. Consequently, as testosterone levels drop, the cell loses ability to generate ATP and a relationship between low testosterone and risk for abnormal insulin sensitivity was demonstrated.
Testosterone has a positive correlation with UQCRB expression and testosterone is required for proper expression of genes that drive ATP that in turn drive the ATP-pump and affect glucose regulation.
Other studies have shown this relationship play out in different ways:
- Among men with DM the frequency of low Testosterone is 20-64% 31
- Of men with type 2 diabetes, 54% had Low Testosterone. The men with lowest testosterone levels demonstrated highest fasting glucose levels compared to men with normal Testosterone.32
- Men with higher testosterone levels had a 42% lower risk of type 2 diabetes. 33
- Total testosterone is inversely related to BMI, body fat ratio, and insulin resistance. 33
- Men with type 2 diabetes have lower testosterone levels than weight-matched non-diabetic control subjects34,35
Hypertension & Testosterone
- There is older literature showing that testosterone has an inverse relationship with blood pressure.36 Last year, Vlachopoulos compared non-diabetic, hypertensive middle aged men to controls and accounted for variables such as age, BMI, smoking and cholesterol. They found that total testosterone is independently and inversely associated with central pulse pressure, wave reflections and left ventricular mass.37
We have the opportunity to see how blood vessels respond to sudden withdrawal of testosterone by following prostate cancer patient who engage androgen deprivation therapy. The study by Smith followed 22 prostate cancer patients as they received androgen deprivation therapy. As testosterone production was blocked (LHRH analog therapy using leuprorelin acetate) the central arterial stiffness was monitored using pulse wave analysis and showed an increase of 21% after just 3 months.38 Of equal interest was that a return to normal was seen in those that discontinued the androgen deprivation therapy. Other findings included a loss of lean body mass while fat mass increased. Shocking changes in insulin levels were seen as insulin rose 28% (11.8 mU/liter up to 15.1mU/liter) in just the first month of therapy and further increase in insulin to 19.3mU/liter was documented after 3 months of therapy. That's a 63.6% rise in insulin production reflecting a stark rise in insulin resistance which directly drives oxidation of LDL and atherosclerotic process.
Lipids & Testosterone
Zhang looked at over 4000 subjects in a retrospective study and saw a clearly evident inverse relationship between testosterone level and LDL.22 They separated 4114 middle aged men by quintiles according to testosterone level. This revealed a clear pattern that as testosterone levels rose, HDL level increased accordingly while LDL and triglyceride levels dropped.
The study by Johnston shows us that the ratio of Oxidized LDL/HDL is one of the best predictors of vascular disease.39 In fact this ratio was 7 times more predictive than LDL level alone. Given that testosterone supports HDL elevation and is also an immune modulator that inversely correlates with oxidized LDL antibodies it would make testosterone a valuable marker for heart disease as well as a therapeutic strategy.
Coronary Artery Disease & Testosterone
The oxidation of LDL is a critical step in atherosclerosis. Macrophages consume oxidized LDL driving foam cell formation. Autoantibodies to oxidized LDL predict:
- Carotid atherosclerosis
- Impairment coronary vasodilation
- Myocardial infarction
- Antibodies to oxidized LDL is the most predictive parameter for extent of coronary involvement. The study by Barud looked at a group of men, 65% of whom had a history of stable coronary artery disease and looked at many variables in search of a correlation to antibodies of oxidized LDL.40 Alteration in serum anti-Oxidized-LDL antibody levels showed no correlation to classical cardiovascular risk factors such as body mass index, waist/hip ratio, smoking, total cholesterol, triglycerides, HDL-cholesterol, or LDL- cholesterol. In multiple regression analysis, only testosterone level was independently associated with anti-Oxidized-LDL antibody levels. This suggests that testosterone is acting as an immune modulator, favorable impacting antibodies that drive atherosclerotic process.
Some of the most compelling evidence that testosterone is key to cardiovascular health is held within the studies showing testosterones impact on cellular energy and GLUT-4 function within the myocyte. Myocardial infarction normally leads to loss of cellular energy within the myocyte. Post infarction is followed by a state of increased insulin resistance, reduced fatty acid oxidation and impaired mitochondrial biogenesis. 41-43
A remarkable study in 2016 by Yang showed that a low testosterone state would predict worse outcomes following myocardial infarction.44 Castration of mice was followed by ligation of a coronary artery to induce cardiac ischemia. The low testosterone state resulted in reduced PPARa activity, increased cardiomyocyte apoptosis and cardiac fibrosis, along with a decrease in ATP. Mice that received testosterone replacement experienced greater cellular energy and cell viability via GLUT-4 protein function, and fatty acid metabolism. Testosterone proved to be supportive of mitochondrial biogenesis and cell recovery.
This is confirmation of a prior study in 2013 by Wilson that demonstrated testosterone
increases GLUT-4-dependent glucose uptake in cultured cardiomyocytes.45 Testosterone increases the cardiomyocytes ability to utilize glucose as a fuel source via the GLUT-4 glucose transporter which becomes critical following ischemic events where the cell has severe energetic needs.
Final comments on testosterones relationship with coronary health include:
- Low T inversely linked to CAD even after adjusting for age and body fat.46
- Men with (+)angiography for CAD had lower T than controls and the degree of coronary involvement correlated inversely with degree of testosterone deficiency. 47
- Administration of T led to coronary dilatation. 48, 49
- Intima media thickness is increased in men with low T.50
- Rotterdam study showed low testosterone levels correlated with increased risk for atherosclerotic disease. 51
Conclusion:
The research of the past 20 years is pointing towards the conclusion that androgens are a key piece of the cardiovascular repair and recovery cycle. Low testosterone levels open the door for inflammation and vascular compromise. When this deficiency is corrected there is a pattern of decreased inflammation, reduced atherosclerosis and resultant drop in CAD. This is not to suggest that healthy testosterone levels are the only key needed for good cardiovascular heath, but it certainly plays an integral part in the process. The literature also dispels the myth that testosterone increases the risk for cardiovascular events. In conclusion, if we are not measuring and treating hypogonadism then we are not offering our patients every available avenue for cardiovascular recovery and good health.
REFERENCES
- Travison, Araujo, et al. A Population-Level Decline in Serum Testosterone Levels in American Men. 2007 The Journal of Clinical Endocrinology & Metabolism 92(1):196–202
- Khurana, S., Ranmal, S., Ben-Jonathan, N., 2000. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperpro- lactinemia and alterations in estrogen receptor expression. Endocrinology 141, 4512–4517.
- Steinmetz, R., Brown, N.G., Allen, D.L., Bigsby, R.M., Ben-Jonathan, N., 1997. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 138, 1780–1786.
- Steinmetz, R., Mitchner, N.A., Grant, A., Allen, D.L., Bigsby, R.M., Ben-Jonathan, N., 1998. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 139, 2741–2747.
- Rubin, B.S., Murray, M.K., Damassa, D.A., King, J.C., Soto, A.M., 2001. Perinatal expo- sure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 109, 675–680.
- Akingbemi, B.T., Sottas, C.M., Koulova, A.I., Klinefelter, G.R., Hardy, M.P., 2004. Inhibi- tion of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroido- genic enzyme gene expression in rat Leydig cells. Endocrinology 145, 592–603.
- Takahashi, O., Oishi, S., 2003. Testicular toxicity of dietarily or parenterally admin- istered bisphenol A in rats and mice. Food Chem. Toxicol. 41, 1035–1044.
- Gupta, C., 2000. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 224, 61–68.
- Welshons, W.V., Nagel, S.C., Thayer, K.A., Judy, B.M., Vom Saal, F.S., 1999. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxy- chlor and other xenoestrogens increases adult prostate size in mice. Toxicol. Ind. Health 15, 12–25.
- Ramos, J.G., Varayoud, J., Sonnenschein, C., Soto, A.M., Munoz De Toro, M., Luque, E.H., 2001. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol. Reprod. 65, 1271–1277.
- Ramos, J.G., Varayoud, J., Kass, L., Rodriguez, H., Costabel, L., Munoz De Toro, M., Luque, E.H., 2003. Bisphenol a induces both transient and permanent histo- functional alterations of the hypothalamic–pituitary–gonadal axis in prenatally exposed male rats. Endocrinology 144, 3206–3215.
- Barret-Connor et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008 Jul; 93(7): 2602–2609.
- Bercea, Patacchiolo, et al. Serum testosterone and depressive symptoms in severe OSA patients. Andrologia. 2012
- Caronia, Dwyer, et al. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin. Endo. 2013, 78:291-296
- Pinto-Fochi, Pytlowanciv, et al. A high-fat diet fed during different periods of life impairs steroidogenesis of rat leydig cells. Reproduction 2016, 152:795-808
- Yuan, Huang, et al. Hyperleptinemia directly affects testicular maturation at different sexual stages in mice, and suppressor of cytokine signaling 3 is involved in this process. Reproductive Biology and Endocrinology 12 15 2014
- Tena-Sempere, Pinilla, et al. Leptin inhibits testosterone secretion from adult rat testis in vitro. Journal of Endocrinology 161 211–218. 1999
- Lin, Vinson & Terracio. Characterization of insulin and insulin- like growth factor receptors in purified Leydig cells and their role in steroidogenesis in primary culture: a comparative study. Endocrinology 119 1641–1647. 1986
- Bebakar, Honour, et al. Regulation of testicular function by insulin and transforming growth factor-beta. Steroids 55 266–269. 1990
- Hammoud, Gibson, et al. Impact of male obesity on infertility: a critical review of the current literature. Fertility & Sterility, Vol 90 No.4 Oct 2008
- Ridker, Rifai, et al. Comparison of C-Reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. NEJM Vol 347, No 20, Nov 2002
- Zhang, Gao, et al. Endogenous sex hormones and C-reactive protein in healthy chinese men. Clinical Endocrinology (2013) 78, 60–66
- Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endo- crinol Metab. 2006;91:345–347.
- Malkin, Pugh, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin. Endocr & Metab 89(7):3313-3318
- Kalinchenko, Tishova, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogondal men with the metabolic syndrome: the double blind placebo controlled Moscow study. Clin Endocrinol (Oxf). 2010 Nov;73(5):602-12.
- Pinderski, Fischbein, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. 2002 Circ Res 90:1064 –1071
- Waehre, Halvorsen, et al. Inflammatory imbalance between IL-10 and TNF in unstable angina potential plaque stabilizing effects of IL-10. 2002 Eur J Clin Invest 32:803– 810
- Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM 2003 Serum level of the antiinflammatory cytokine interleu- kin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 107:2109 –2114
- C. J. Malkin, P. J. Pugh, P. D. Morris, S. Asif, T. H. Jones, and K. S. Channer, “Low serum testosterone and increased mortality in men with coronary heart disease,” Heart, vol. 96, no. 22, pp. 1821–1825, 2010
- Pitteloud, Mootha, et al. Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men. Diabetes Care 2005 Jul; 28(7): 1636-1642
- Dhindsa, J.Clin.Endocrin.Metab 2004
- Corrales, Partial Androgen Defic., Metab. 2004
- Ding, Sex differences in endogenous sex hormones, JAMA 2006
- Barrett-Connor E: Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med, 1992]
- Andersson B, Marin P, et al: Testosterone concentrations in women and men with NIDDM. Diabetes Care, 1994
- Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens. 1988;6:329–332.
- Vlachopoulos, Pietri, et al. Inverse association of total testosterone with central haemodynamics and left ventricular mass in hypertensive men. Atherosclerosis 250 (2016) 57e62
- Smith JC, Bennett S,et al. . The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86: 4261– 4267.
- Johnston, Jernberg, et al. Improved Identification of patients with coronary artery disease by the use of new lipid and lipoprotein biomarkers. Am.J.Cardiology 2006
- Barud, Palusinskia, et al. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis 164 (2002) 283/288
- Amorim, Nguyen, Shingu et al., “Myocardial infarc- tion in rats causes partial impairment in insulin response asso- ciated with reduced fatty acid oxidation and mitochondrial gene expression,” Journal of Thoracic and Cardiovascular Surgery, vol. 140, no. 5, pp. 1160–1167, 2010.
- Heather, Cole, Lygate et al., “Fatty acid trans- porter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart,” Cardiovascular Research, vol. 72, no. 3, pp. 430–437, 2006.
- Rosenblatt-Velin, Montessuit, Papageorgiou, et al. “Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism,” Cardiovascular Research, vol. 52, no. 3, pp. 407–416, 2001.
- Yang, Wang, et al. Testosterone replacement modulates cardiac metabolic remodeling after myocardial infarction by upregulating PPARalpha. PPAR Research, Vol 2016
- Wilson, Contreras-Ferrat, et al. Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. Cellular Physiology 2013
- Phillips, Pinkernell, et al. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994 May;14(5):701-6.
- Rosano, Sheiban, et al. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007 Mar-Apr;19(2):176-82. Epub 2006 Aug 31.
- Webb, McNeill, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1999;100:1690-1696
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94: 2614 –2619.
- Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079.
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87: 3632–3639.





